Table of Contents
- Meta-Analysis Reveals 12.2% Average Weight Loss Throughout Researches
- Adverse Effects Significantly Reduced vs Other Balloons
- Reference:
Elipse Swallowable Intragastric Balloon is an alternative option for overweight patients who seek a supportive treatment besides the dietary changes. The Allurion intragastric balloon (formerly the Elipse, Allurion Technologies), an unique balloon that is swallowed, calling for no surgical procedure or endoscopic positioning, shows high efficacy in attaining fat burning as well as an enhanced metabolic profile, with fewer unfavorable events than reported with various other available gastric balloons, arises from a meta-analysis show.

This research is the most thorough evaluation of the Allurion balloon and released in the November/December 2021 issue of the Journal of Scientific Gastroenterology. This study revealed that the Allurion balloon decreases waist circumference as well as triglyceride levels and also is related to less unfavorable effects when compared with various other intragastric balloons, the writers wrap up.
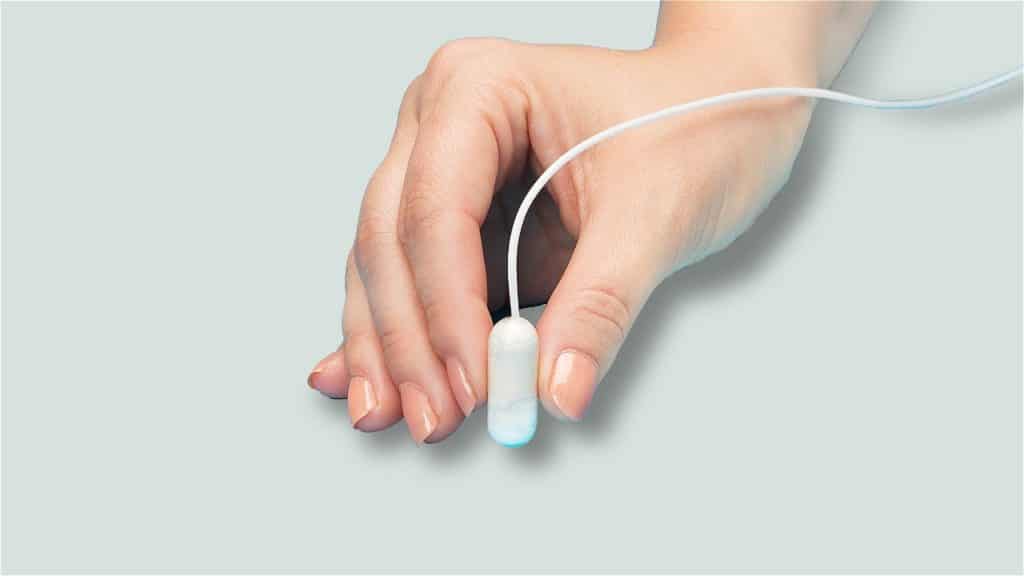
Unlike other balloons, the Allurion swallowable stomach balloon is compressed into a little capsule that is linked to a slim catheter and also, once swallowed, it is after that filled up with 550 mL of fluid through the catheter to produce a sensation of fullness as well as assist control cravings.
The treatment can be carried out on an outpatient basis in approximately 20 mins, possibly preventing the problem and extra expenses of surgery or endoscopic positioning and elimination. After approximately 4 months, the balloon is designed to empty with a valve that spontaneously opens up, as well as the balloon is then passed by the stool.
Though currently used and succeded thousands of cases around the globe, the balloon does not yet have authorization from the United States FDA.
Meta-Analysis Reveals 12.2% Average Weight Loss Throughout Researches

To evaluate the balloon's performance, the authors recognized 7 out of 273 released researches that fulfilled the analysis standards. The research studies included 2152 individuals, varying in age from 18 to 65 years, with a mean standard body mass index (BMI) of 32.1 to 38.6 kg/m2.
Every one of the research studies were potential, with reported end results at 3 to 4 months, when the Allurion balloon typically decreases. 3 of the studies were multicenter, while 4 were single-center.
In regards to improvements in BMI, the average percentage of complete body weight reduction throughout treatment throughout the research studies was 12.2%. The mean excess body weight reduction throughout the Allurion research studies was 49.1%.
The analysis was not created to straight contrast end results with other balloons, yet the writers keep in mind, for example, that the ReShape Duo intragastric balloon (an FDA-approved dual-balloon system) has actually been reported in a previous research study to be connected with a portion of total body fat burning of 7.6% at 6 months, compared to 3.6% observed among those with way of life alterations.
Nevertheless, a different meta-analysis showed the pooled percent of overall body weight management with the FDA-approved Orbera balloon to be concerning the same as the existing Allurion evaluation, at 12.3% at 3 months after implantation (adhered to by 13.2% at 6 months and 11.3% at year).
That analysis better revealed excess body fat burning with the Orbera balloon at 12 months to be 25.4%. In various other results, the present meta-analysis likewise showed considerable renovations with the Allurion balloon in waistline area of 0.89 (P =.001) and also in triglyceride levels of 0.66 (P =.004) versus baseline.
Previous research involving the FDA-approved Obalon intragastric balloon, which is inflated with gas rather than fluid, revealed a substantial reduction in midsection circumference from 109 centimeters ( ± 12.3) to 99 centimeters ( ± 10.5) (P <.05), and one more research study revealed that 37.5% of clients receiving the Orbera balloon had actually stabilized triglyceride levels after 4 months, without concomitant clinical treatment.
Adverse Effects Significantly Reduced vs Other Balloons
Possible risks connected with the Allurion balloon include the possibility for very early depreciation; nevertheless, the pooled rate of very early balloon deflation observed in the meta-analysis was fairly reduced, at 1.8%.
Other side effects reported with the Allurion balloon were stomach discomfort (37.5%), throwing up (29.6%), diarrhea (15.4%), as well as little bowel blockage (0.5%).
The equivalent prices of stomach pain with the ReShape Duo as well as Orbera balloons have been reported at 54.5% and also 57.5%, respectively, with the effects potentially because of overinflation, the writers note.
And also prices of throwing up with the ReShape Duo as well as Orbera balloons have actually been reported as much greater, at 86.7% and also 86.8%, respectively.
Of note, there were no deaths or cases of severe pancreatitis reported in the meta-analysis researches of Allurion.
As reported by Medscape Medical News, such concerns have actually been elevated in previous FDA notifies regarding the Orbera and also Improve Duo liquid-filled intragastric balloons.
Theoretically, because the Allurion balloon is positioned for a much shorter time span, it is conceivable that there might be much less severe side effects. However, comparative trials are needed to verify this.

Although the balloons reveal effectiveness in patients having problem with weight management, metabolic syndrome, and fatty liver disease, "the type and also period of intragastric balloons ought to be tailored to the person,"
" Medical professionals ought to extensively go over with their people the advantages and threats of using an intragastric balloon," Moreover, positioning of intragastric balloons must just be tried by clinicians with knowledge in bariatric endoscopy.
Reference:
1-Swallowable Intragastric Balloon Shows Significant Weight Loss - Medscape - Jan 12, 2022
2-J Clin Gastroenterol. 2021; 55:836 -841

